The pharmaceutical industry is one of the most innovative and biggest-selling sectors in the world. In 2024, the European pharmaceutical market generated sales of around 280 billion US dollars. In Germany alone, sales in the first quarter of 2025 totalled around 16.6 billion euros, making the country the largest pharmaceutical market in Europe and the fourth largest in the world. Alongside this, packaging technology is becoming more important: it is not only a means of safely storing and transporting medicines, but is also a key issue where sustainability is concerned.
When it comes to packaging, regulation plays a central role in the pharmaceutical industry; the Packaging & Packaging Waste Regulation (PPWR) is going to concern the pharmaceutical and medical technology sector, too, in the long term. Although the regulation does not initially apply to the packaging of medical products and pharmaceuticals – transitional periods apply here until 2035 and 2040, respectively – the industry needs to take action now. The intensity with which it is already working on solutions will also be evident at interpack 2026 – from recyclable blister materials to digitalised production lines.
Recyclable materials for pharmaceutical products
More attention is therefore being paid to sustainability. Some solutions – such as reusable and refillable packaging – will be difficult to realise for pharmaceuticals, as will the use of recyclates, which is subject to strict regulatory limits. In the area of primary packaging, however, packaging and film manufacturers have already developed a range of sustainable alternatives in recent years, for example for standard blister packs made of PVC and aluminium. Initial solutions are already on the market: moulded film and lidding film are made of PET or PP, or even paper-based materials with a barrier.
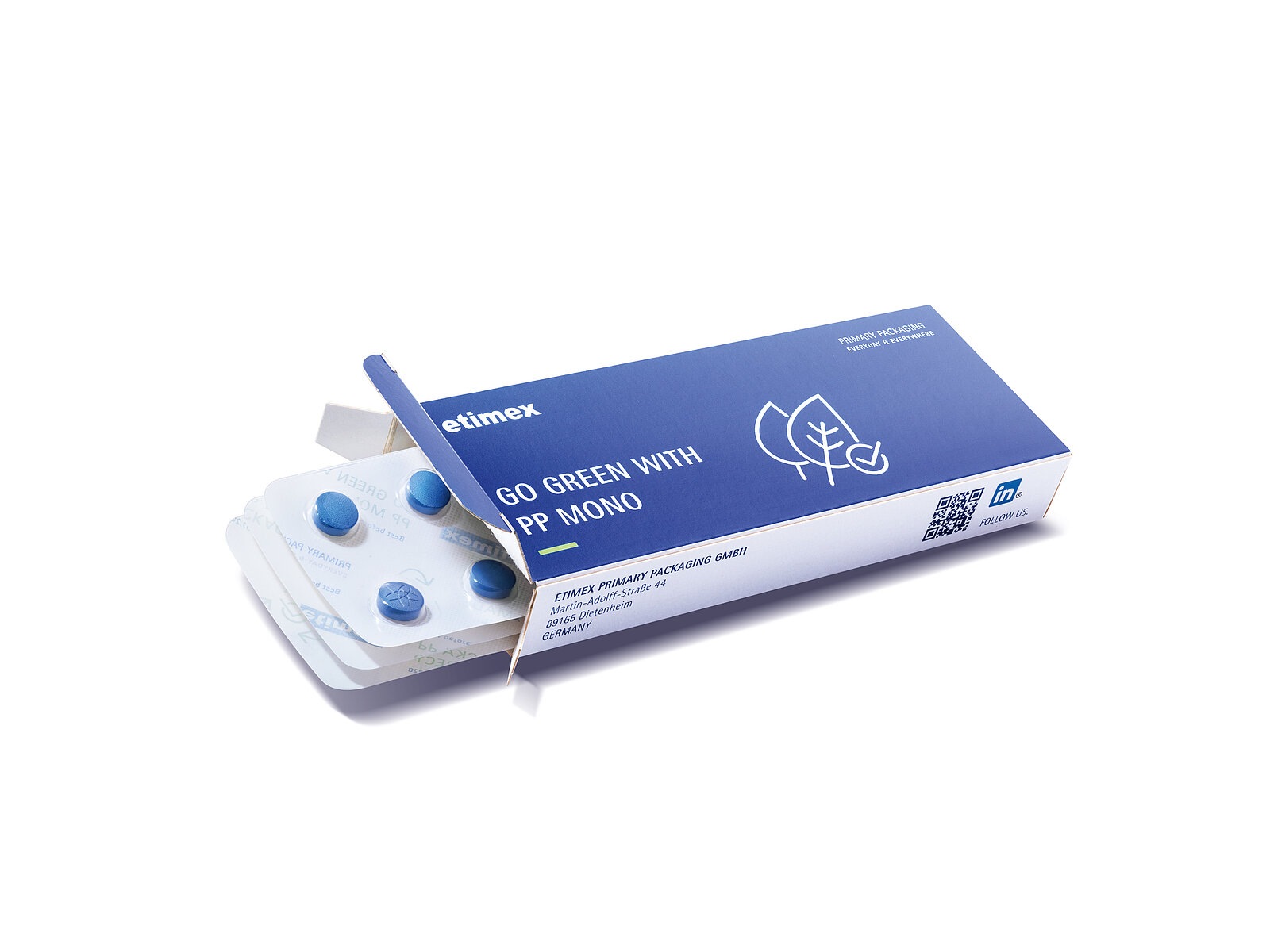
In a joint project, four packaging companies have developed a polypropylene pharmaceutical blister pack that meets the strict
requirements of the pharmaceutical industry. The material from PP blister film manufacturer Etimex was tested and optimised for practical suitability under realistic conditions on a modern blister machine from interpack exhibitor Uhlmann Pac-Systeme.
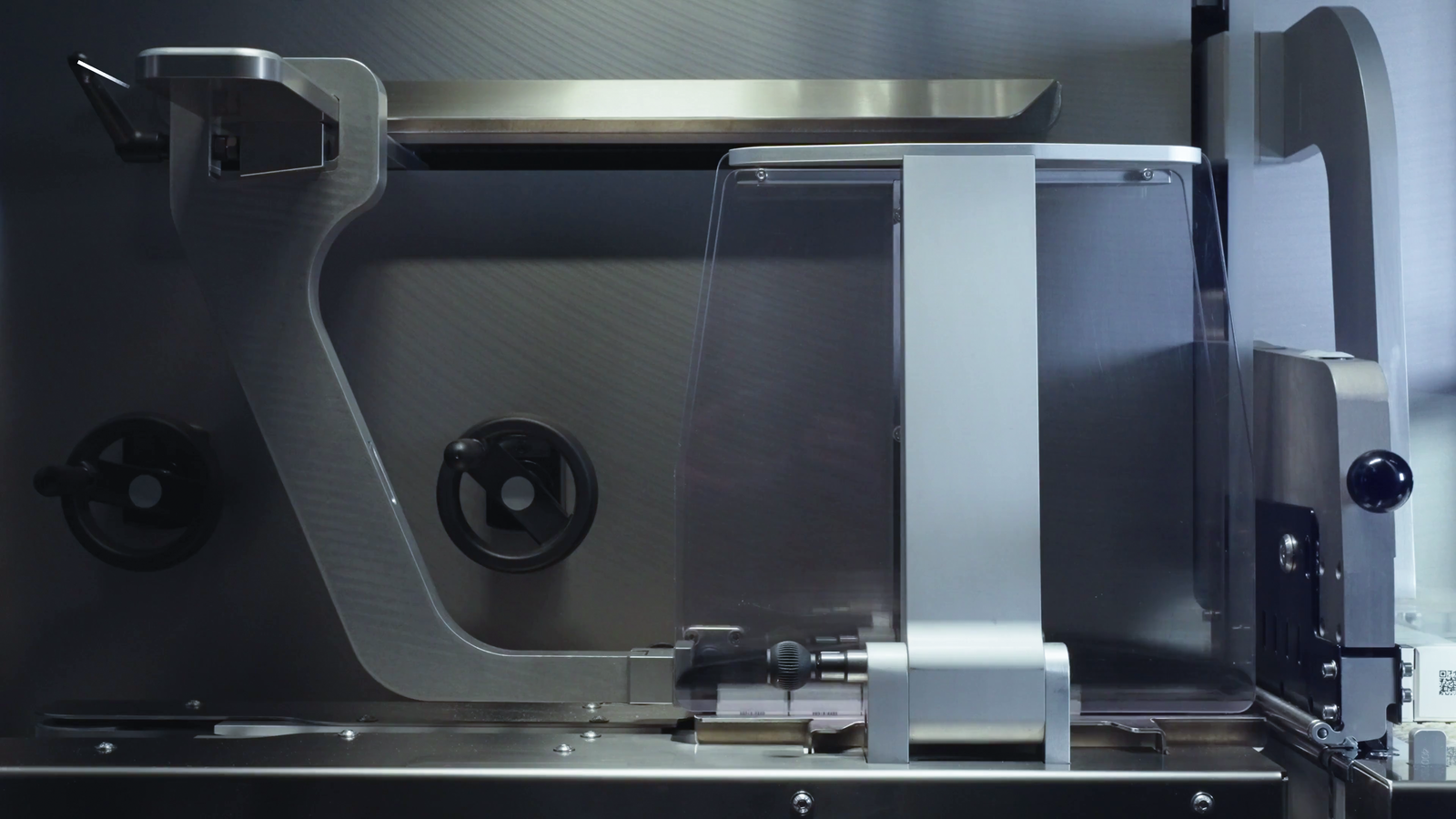
Many machine manufacturers have been busy focussing on the processing of sustainable packaging materials. Romaco produces blister packs made of recyclable PET monomaterial on its new Noack N 760 plate sealing machine. It achieves a maximum output of up to 150 blisters per minute when producing PET/PET blisters in four-lane operation. The universally applicable intermittent blister machine is suitable for packaging a very wide range of products, from solid dosage forms, ampoules and medical products through to semi-solid forms.
Digitalisation and robotics in the pharmaceutical process
The latest machine developments are characterised by modularity, automation and robotics. Many of these technologies were first presented to a wide audience of professionals at interpack and have been shaping production processes ever since.
Steriline technology ensures safe primary packaging for sensitive medicines. The company combines robotics with a 3D vision system (3D CPS) to reduce particle emissions and enable flexible, low-contamination production processes. The system recognises objects in the work area and adjusts its movements in real time, allowing for the replacement of conventional feeding systems such as hoppers or vibrating bowls. The 3D CPS was developed together with ISS – Innovative Security Solutions, a spin-off of the Politecnico di Milano. With this collaboration, the two companies aim to combine pharmaceutical process expertise with state-of-the-art robotics and image processing.
Christ Packing Systems is also focussing on innovation. The Ottobeuren-based company has been granted a patent for a new type of sealing unit developed specifically for the requirements of the pharmaceutical and medical technology sectors. Unlike conventional machines, which seal from below, the FilmTeq 250 seals from above. According to the manufacturer, this means that the sealing table remains unheated, which prevents the product from heating up and reduces the amount of cooling required. The top-down process also enables a more compact machine design with a smaller footprint.
Mechanical engineering companies such as Syntegon benefit greatly from the dynamics of the pharmaceutical market. In 2024, the company achieved record results driven by the increasing demand for complete aseptic solutions. This year, the system provider presented a new line concept for liquid pharmaceuticals, which was developed together with two partners from the pharmaceutical industry. SynTiso ensures faster aseptic transportation and up to 50 per cent shorter batch changes in a smaller space. With 100 per cent in-process control, up to 600 syringes, vials or cartridges can be processed per minute – a speed never before seen on the market, according to Syntegon. This is an important factor in vaccine production, among other manufacturing processes. The system is also suitable for filling ready-to-use (RTU) containers.
Smart packaging: from NFC to functional labels
In addition to the ecological dimension, digital and functional added value continue to take centre stage. Packaging is increasingly being equipped with RFID chips, NFC labels and battery-free Bluetooth sensors to obtain real-time data on the location, temperature or condition of the packaging. Smart blisters can do even more: their sensors register the removal of individual tablets and thus record the dosing times. Security labels such as Securikett’s paper-based VOID labels offer important and sustainable tamper protection. In addition to tamper protection, they can be recycled together with the folding box they seal. Using electronic package leaflets not only saves paper, but also makes it easier for consumers to access information.
Interpack 2026 will once again showcase customised solutions and innovative concepts along the entire pharmaceutical packaging value chain.
One thing is certain: companies that invest in research, a digital infrastructure and recyclable materials today will secure competitive advantages in the long term. In future, it will be less about the active ingredient itself and more about how safely, sustainably and intelligently a medicine reaches the patient.